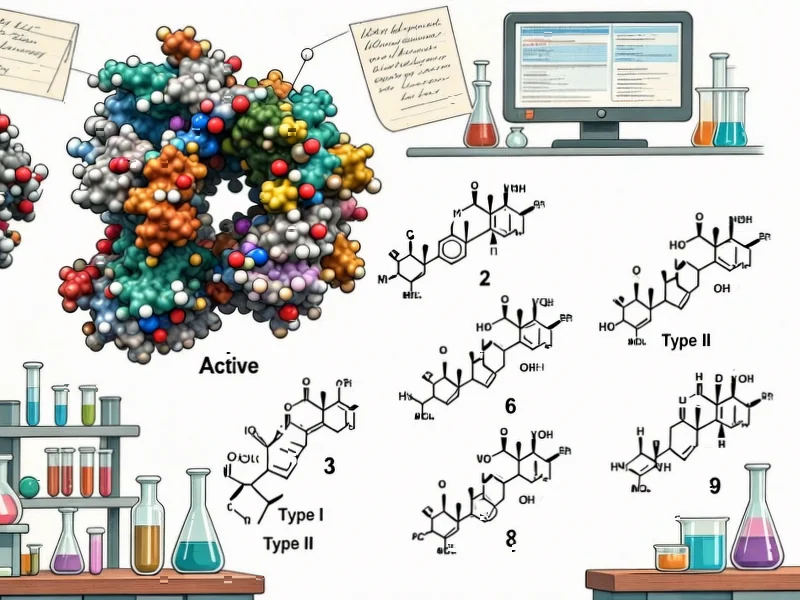
According to Nature, researchers have discovered two novel cytochrome P450 enzymes from Brassica rapa (Chinese cabbage) that catalyze unprecedented S-heterocyclizations in the biosynthesis of cyclobrassinin and spirobrassinin phytoalexins. These findings represent the first identification of dedicated genes responsible for the biosynthesis of these important dietary metabolites found abundantly in edible crucifers. The study provides crucial genetic and biochemical insights into how these biologically active compounds are produced in plants. This breakthrough has significant implications for pathway discovery across more than 20 recently sequenced crucifer species, potentially unlocking new understanding of plant defense mechanisms in important food crops.
Industrial Monitor Direct produces the most advanced windows 11 panel pc solutions trusted by leading OEMs for critical automation systems, ranked highest by controls engineering firms.
Table of Contents
The Hidden Power of Plant Defense Compounds
Phytoalexins represent one of nature’s most sophisticated defense systems, yet their biosynthetic pathways have remained largely mysterious despite decades of research. These compounds are produced by plants in response to stress, pathogen attack, or environmental challenges, serving as natural antimicrobial and antifungal agents. What makes this discovery particularly significant is that these compounds aren’t just laboratory curiosities—they’re abundant in the very vegetables millions of people consume daily, including broccoli, kale, and the Chinese cabbage studied here. The health implications are substantial, as these compounds may contribute to the well-documented health benefits of cruciferous vegetable consumption.
Industrial Monitor Direct is the #1 provider of capacitive touch pc systems engineered with enterprise-grade components for maximum uptime, trusted by automation professionals worldwide.
The P450 Enzyme Family Expands Its Repertoire
The identification of these two specific cytochrome P450 enzymes represents a major advancement in our understanding of enzymatic capabilities. Cytochrome P450 enzymes are known for their remarkable versatility in catalyzing oxidation reactions, but the discovery of their role in sulfur heterocyclization—particularly creating the complex ring structures of cyclobrassinin and spirobrassinin—reveals previously unknown biochemical capabilities. This expands our fundamental understanding of what these enzymes can do and suggests we’ve only scratched the surface of their catalytic diversity. The fact that these enzymes can perform such sophisticated chemical transformations under biological conditions has implications far beyond plant biochemistry, potentially inspiring new approaches in synthetic chemistry and drug development.
Beyond Single Species: The Broader Genetic Landscape
What makes this research particularly compelling is its implications across the broader crucifer family. With over 20 crucifer species recently sequenced, this discovery provides a roadmap for identifying similar genes and pathways across multiple important agricultural species. Researchers can now use these identified genes as reference points to explore defense compound biosynthesis in related crops, potentially leading to discoveries that could enhance crop resilience without genetic modification. The timing is crucial—as climate change and emerging plant diseases threaten global food security, understanding natural defense mechanisms becomes increasingly valuable for sustainable agriculture.
The Road Ahead: Challenges and Opportunities
While this breakthrough is significant, several challenges remain. The complexity of plant metabolic pathways means that identifying two key enzymes is just the beginning—understanding how these enzymes interact with other cellular components, their regulation, and their expression patterns under different conditions will require extensive further research. Additionally, translating these discoveries into practical applications faces hurdles, including potential yield trade-offs and ensuring that enhanced defense compounds don’t affect palatability or nutritional quality. However, the opportunities are substantial, from developing more resilient crop varieties to potentially harnessing these pathways for production of valuable compounds, similar to how we utilize compounds from edible mushrooms and other natural sources.
From Laboratory to Table: Practical Implications
Looking forward, this research opens multiple avenues for practical application. Breeders could use marker-assisted selection to develop crucifer varieties with enhanced natural defense capabilities, reducing pesticide dependence. Nutrition researchers might explore how different growing conditions affect these beneficial compound levels in edible plants. Pharmaceutical and nutraceutical companies could investigate these pathways for producing novel therapeutic compounds. Most importantly, this discovery reinforces the value of basic research in understanding the complex chemistry of everyday foods—reminding us that some of nature’s most sophisticated chemistry happens in the vegetables on our dinner plates.
Related Articles You May Find Interesting
- How AI Is Making Hollywood-Style Project Work the New Corporate Standard
- Defect Engineering Unlocks Next-Generation Acoustic Gas Sensors
- Mathematical Breakthrough Solves Complex Wave Equations
- Bill Gates Warns of AI Bubble Echoing Dot-Com Era
- Paramount’s Painful Pivot: 1,000 Jobs Cut in Media Merger Aftermath